
Details
ELISA Kit for the Quantitative Measurement of E. coli HCP Residues in Protein Purification Process and End-Product
**1. APPLICATION**
The kit is intended for the quantitative determination of residual host cell protein in protein therapeutics expressed by E. coli expression systems.
**2. BACKGROUND**
Escherichia coli, commonly known as E. coli, is widely utilized as an expression system. During the process of extracting target proteins from fragmented bacterial cells, a substantial portion of host proteins is released along with the target protein. These host proteins exhibit strong immunogenicity, which can lead to adverse toxicity or immune reactions, jeopardizing product safety and quality, and causing potential biological contamination. One of the aims of downstream processes in the production of biological medicinal products is to remove these potential hazards.
Therefore, it is essential to minimize the residual levels of host cell proteins (HCP), and in the downstream purification process development, a scientifically sound method for determining the concentration of HCP in finished or semi-finished products is necessary. Enzyme-linked immunosorbent assay (ELISA) has a high sensitivity and is therefore designated as the gold standard for HCP detection by regulatory agencies.
**3. MATERIALS (Note: Storage at 2-8℃)**
Reagents
Specification
Quantity
1
Pre-Coated Microplate
(Detachable)
48 wells
1 plate
(Keep Sealed)
2
Standard (Stock Solution) -500ug/ml
30ul
1 tube
3
Detection antibody (100×)
100ul
1 tube
4
TMB Substrates
6ml
1 vial (Avoid Light)
5
Stop Solution
6ml
1 vial
6
Wash Solution (100×)
10ml
1 vial
7
Diluent Buffer (10×)
10ml
1 vial
8
Plate Sealer
4 pieces
9
Instruction Manual
1
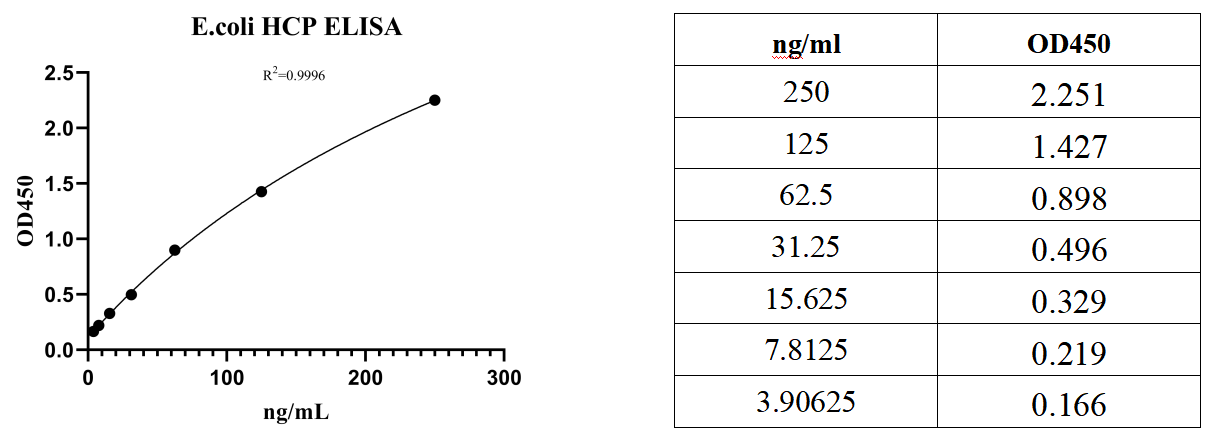
Appendix 1: example OF Standard Curve
Partial purchase records(bought amounts latest0)
User Comment(Total0User Comment Num)
- No comment


 +86 571 56623320
+86 571 56623320




